
Elevate Your Audiology Clinic and Research Lab with State-of-the-Art Solutions
Mimosa Acoustics is dedicated to providing equipment that improves and simplifies hearing screening and diagnostics. Our goal is to offer your patients and research participants the best science has to offer in a user-friendly clinical package. OtoStat and HearID allow you to test middle and inner-ear status quickly and easily with a single probe fit.
We support both clinicians and researchers, providing easy-to-use interfaces with pre-programmed protocols that are also highly customizable.
- Flexible customization: Tailor protocols to specific needs, from routine screenings to advanced research.
- Rapid assessments: Quickly obtain middle- and inner-ear test results for efficient patient care and research.
- Precise, reliable measurements: Acquire accurate data for in-depth analysis.
- User-friendly interface: Streamlined workflows to maximize efficiency.
Get in touch to discuss your needs and receive a free quote.
OtoStat® 3.3
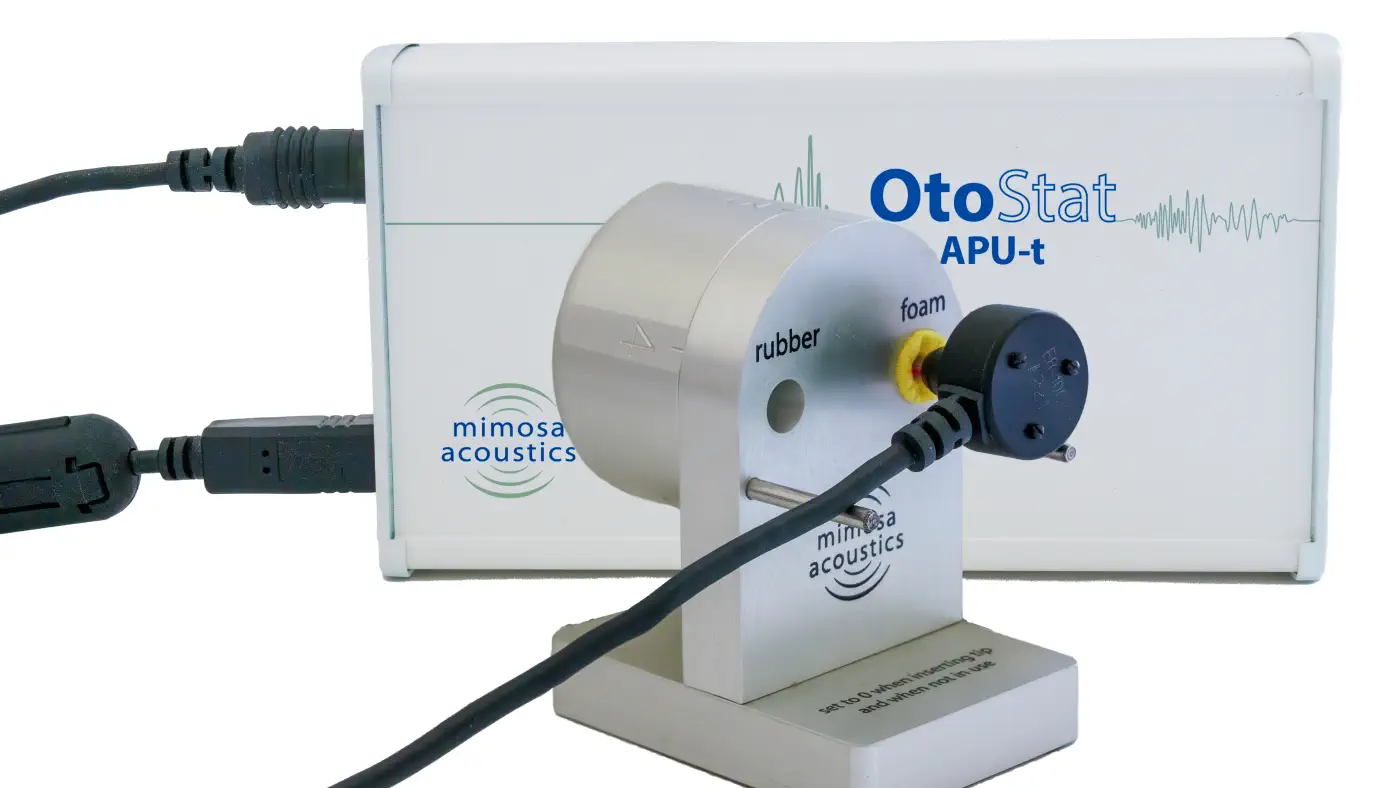
OtoStat is a portable, lightweight USB device for testing DPOAE and wideband acoustic immittance (MEPA) quickly and easily. OtoStat systems include OtoStation® patient management and testing software.
The monaural OtoStat is an FDA 510(k) legally marketed device for MEPA and DPOAE.
For research users, get accurate stimulus levels with forward pressure level calibration. Add TEOAE, SFOAE, ipsilateral MEMR, Swept DPOAE, and Swept SFOAE to the monaural system, or choose the binaural OtoStat to also get contralateral MOCR, binaural/contralateral MEMR, PTA, and access to GPIO lines.
HearID® 5.1

HearID incorporates portable, lightweight hardware with flexible, intuitive software. The integrated user interface gives instant access to patient records and the customizable measurement modules, which include wideband acoustic immittance (MEPA), DPOAE, and TEOAE otoacoustic emission modules). HearID is a legally marketed FDA 510(k) device.
Clinician's Guides
Adults
 Differential diagnosis of conductive hearing loss.
Differential diagnosis of conductive hearing loss.
Children
 Identify middle-ear effusion in children.
Identify middle-ear effusion in children.
Infants
 Identify conductive hearing loss in infants.
Identify conductive hearing loss in infants.
 Newborn/Infant Hearing Screening Programs
Newborn/Infant Hearing Screening Programs